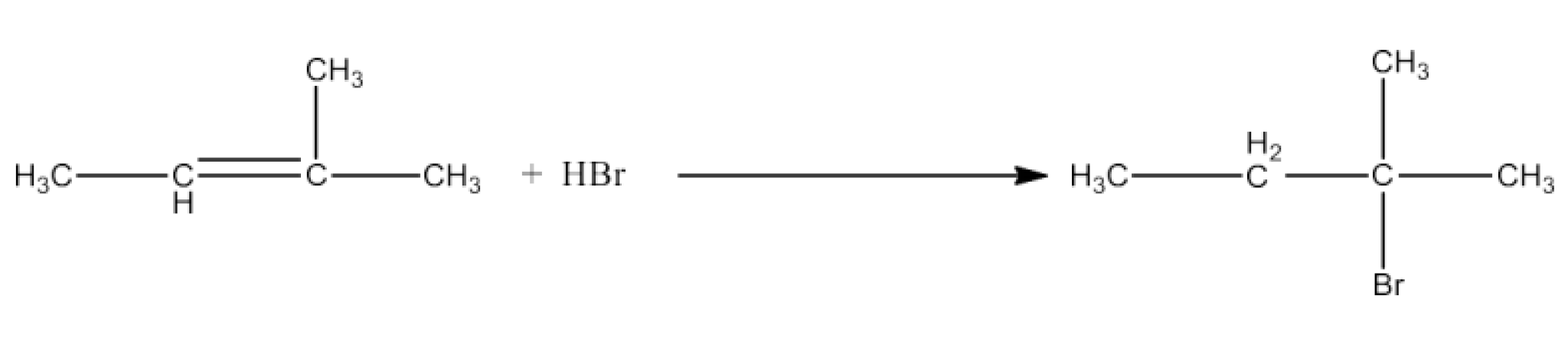Haloalkanes and Haloarenes Class 12 NCERT Solutions FREE PDF Download
FAQs on NCERT Solutions for Class 12 Chemistry Chapter 6 Haloalkanes and Haloarenes
1. What step-wise method should be followed to solve NCERT questions related to Haloalkanes and Haloarenes in Class 12 Chemistry?
To solve NCERT problems on Haloalkanes and Haloarenes systematically, students should:
- Identify the type of organic compound and its functional group.
- Recall relevant reaction mechanisms such as substitution (SN1/SN2) or elimination (E1/E2).
- Apply stepwise IUPAC naming conventions for structures involved.
- Follow the correct sequence of conversions using CBSE-specified reagents and conditions.
- Write balanced equations for each step as per CBSE format.
- Draw structures for intermediates as required to support your answer.
2. How do NCERT Solutions for Class 12 Chemistry Chapter 6 help clarify the difference between SN1 and SN2 mechanisms?
These solutions provide detailed, step-by-step explanations for both mechanisms:
- SN1 reactions proceed through carbocation formation, favored by tertiary halides in polar protic solvents and result in partial racemization.
- SN2 reactions occur in a single step, favored by primary halides in polar aprotic solvents and lead to complete inversion of configuration (Walden inversion).
- NCERT Solutions illustrate real examples for both, aiding conceptual distinction.
3. Why are haloalkanes generally more reactive than haloarenes according to NCERT Solutions?
Haloalkanes are more reactive because the carbon-halogen bond (C–X) is on an sp3-hybridized carbon, making it more polar and weaker. In haloarenes, the halogen attaches to an aromatic ring (sp2 carbon), and resonance delocalization strengthens the bond, making nucleophilic substitution much more difficult.
4. What are the common misconceptions students face regarding elimination and substitution in haloalkanes, and how are these addressed?
Typical misconceptions include:
- Assuming elimination and substitution are mutually exclusive (both can happen based on solvent and base).
- Overlooking rules like Saytzeff’s and Hofmann’s for major alkene products.
- Misapplying Markovnikov's rule in the presence of peroxides (anti-Markovnikov addition may occur).
5. How do electron-withdrawing and electron-donating groups affect the reactivity of haloarenes compared to haloalkanes?
In haloarenes, electron-withdrawing groups (like NO2) in ortho/para positions increase reactivity towards nucleophilic substitution, while electron-donating groups (like –OH, –NH2) decrease it by stabilizing the aromatic ring. In haloalkanes, such substituent effects are minor since no aromatic resonance is involved.
6. What are ambident nucleophiles and how does the NCERT Solution explain their behavior in organic reactions?
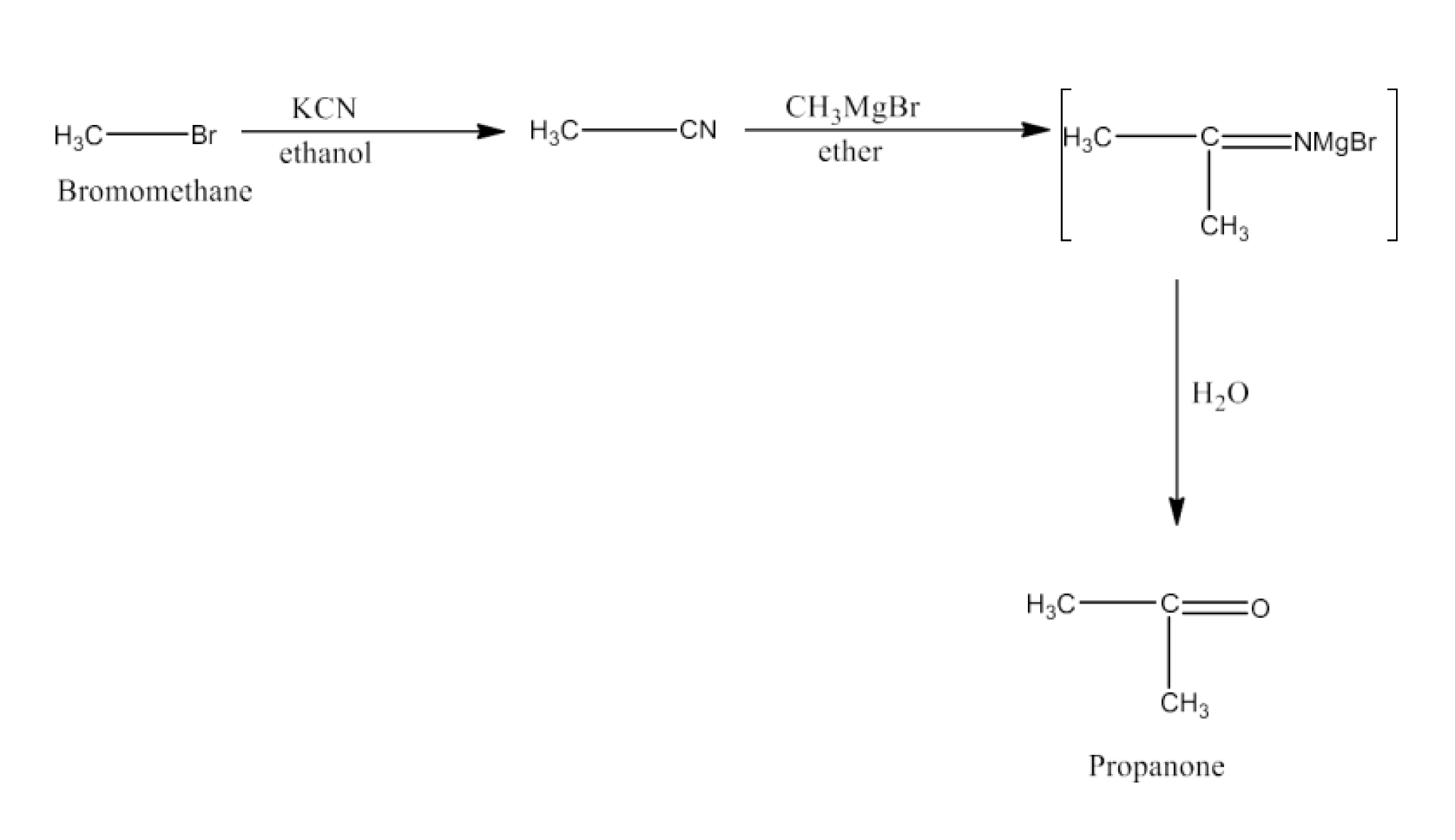
An ambident nucleophile can attack through two different atoms; for example, cyanide ion (–CN) can bond through C or N. NCERT clarifies that alkylation with KCN yields nitriles (C-attack), while AgCN gives isonitriles (N-attack), with reasoning linked to nucleophile structure and reaction conditions.
7. Why are alkyl halides insoluble in water even though they are polar molecules?
Although alkyl halides are polar and have dipole–dipole interactions, they cannot form hydrogen bonds with water. The energy required to disrupt the strong water lattice is not compensated by new attractions, resulting in low solubility despite their polarity.
8. What is the importance of preparing Grignard reagents under anhydrous conditions, as mentioned in Class 12 Chemistry NCERT Solutions?
Grignard reagents are highly reactive with moisture; if water is present, they instantly form alkanes, destroying the reagent. NCERT emphasizes that absolute dryness is crucial to maintain reagent activity for further synthetic reactions.
9. How are environmental effects of organohalogen compounds such as DDT and Freon-12 discussed in NCERT Solutions?
NCERT Solutions highlight that organohalogens like DDT accumulate in fat tissues, leading to ecological harm and resistance, while Freon-12 used as a refrigerant and propellant contributes to ozone layer depletion. These points align with board questions on real-world chemical impacts.
10. Which method should be used to systematically name and draw structures of halogenated organic compounds as per NCERT guidelines?
To write IUPAC names and structures, the method involves:
- Identifying the longest carbon chain and all functional groups.
- Numbering so substituents get the lowest possible locants.
- Listing halogens and alkyl groups alphabetically.
- Drawing clear, labeled structures showing all positions distinctly.
11. What is the role of resonance in the chemical properties of haloarenes compared to haloalkanes?
Resonance in haloarenes involves delocalization of the halogen's lone pairs into the aromatic ring, strengthening the C–X bond and making nucleophilic substitution more difficult. In haloalkanes, no such resonance exists, resulting in higher reactivity.
12. How do NCERT Solutions for Chemistry Chapter 6 guide students in multi-step organic conversions?
Solutions break down complex conversions (e.g., ethanol to but-1-yne) into discrete, logical steps detailing each intermediate compound and the required reagents/conditions, using official CBSE reaction pathways. This approach teaches students to analyze target product and work backwards, which is essential for board and entrance exams.
13. Why is detailed stepwise working emphasized in NCERT Solutions for solving Chemistry Chapter 6 problems?
Detailed, step-by-step solutions ensure conceptual clarity, allow students to follow logical problem-solving techniques, and match the marking schemes used in CBSE board exams. This also helps avoid missing steps that could result in loss of marks.
14. How can using NCERT Solutions for Class 12 Chemistry Chapter 6 help in preparing for both board and competitive exams?
- Covers the full CBSE 2025–26 syllabus depth necessary for board questions.
- Provides advanced application and HOTS questions relevant for competitive exams like JEE/NEET.
- Focuses on mechanism clarity, named reactions, and multi-step organic conversions—highly examinable in both contexts.
15. What advanced application questions does NCERT Solutions address in the context of haloalkanes and haloarenes?
High-order thinking problems include predicting major/minor products when elimination and substitution compete, synthesizing target molecules in multiple steps, rationalizing the order of boiling points or dipole moments, and comparing the reactivity of different isomers in substitution reactions—all explained stepwise for exam mastery.